Questions related to Mice
Hello,
I am thinking to implant glass cannulas (200 micrometers diameter) bilaterally just above the Ventral Tegmental Area in mice.
Former colleagues tried, but encountered high mortality immediately post-op (within 24 hours from implantation). Anybody had a similar issue while implanting on VTA? Any suggestion before I try myself?
Here's the coordinates I've been recommended:
AP: -3
ML: 1.15
DV: -4.2
10° angle.
Thanks for sharing your experience!
Hi everyone,
I'm going to generate CAR-T cells starting with mouse CD3+ T cells. I isolated mouse CD3+ T cells using Miltenyi Pan T cell isolation kit. I seeded cells 1millon/well in 24 well plate precoated with 1ug/ml antiCD3 and 2ug/ml antiCD28. Cells were culture in the presence of 50IU/ml rhIL-2. However, only 20% of the cells were alive after 48h culture. Not a lot of cells became bigger, and I didn't see clusters forming. My question is that is it because of not sufficient TCR stimulation or AICD? I'm using RPMI supplement with PS, Glutamax, sodium pyruvate, 10% FBS, b-Me. Thank you in advance!
I am currently working towards developing a model for type 2 diabetes, using fructose and streptozotocin (STZ). Prior to injecting my C57BL/6 mice with STZ, we tested blood glucose with a tail-prick, using an AccuChek Instant glucometer. As per methods used in relevant literature, I fasted my mice for 4 hours prior to checking blood glucose (BG). The mean BG in my normal control, which did not receive fructose water was around 8 mmol/L, a BG akin to mild hyperglycaemia. Bear in mind, this group is not due for induction, and received a normal diet of commercially available mice pellets. For the second BG test, I fasted my animals for 6 hours, and got the same if not similar results.
Please assist.
Hi.
I am currently trying to isolate Neutrophils from mouse blood. I am using histopaque 1077 and 1119. I have followed the protocol set out by Sigma, which involves layering histopaque 1119 and 1077, followed by the whole blood. I spined it for 30 min at 700g.
The PBMC layer is evident, but weren't able to see the neutrophil layer (see attached image). The RBC don't seem to sediment. I had tried the same protocol using human blood and it worked. I'm not sure why it isn't working with the mice blood.
Can anyone give any tips or recommendations on isolating neutrophils from mouse blood.
Thank you:)

We are isolating total RNA from mouse sceletal muscle, using the beads beater with the sample already being in Trizol, afterwards chloroform extraction and finally Qiagen RNeasy kit. On the TAPE station we see a nice sharp 18S RNA band, but absolutely no 28S RNA. Does anyone have an idea what the problem may be?
I need to determine the area of the ossified regions and the ossification percentage, but to do that accurately, I first need to measure the total bone area. I'm using Fiji software for this task, but I'm uncertain about the most accurate method for measuring the entire bone length. and also the length that Fiji provides has no unit what is exactly the unit that it calculates?
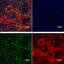
As a complete newbie to retina research (and imaging in general) - I'd be grateful if anyone could look at this image and offer insight.
This slice of a mouse eye shows two fluorescently labeled proteins (green and red)(x10 objective on slide scanner).
Note the proteins were introduced by viral injection and the red protein should be synaptic.
My intuition is that the red fluorescence here is artefact - due the signal in the choroidal stroma (TOP band) and the diffuse blurry pattern of all red bands. However the signal in what I take to be OPL and IPL is consistent with where this protein might be found.
Perhaps this pattern is autofluorescence of some kind common to these retinal layers? Any thoughts would be appreciated
Thanks
Tommy


Please help! cant find any pictures on the web with the same paterns

Hi everyone,
I was wondering can we differentiate Treg from tissue, like brain tissue or tumor just by the markers CD4+ CD25+ and Foxp3+ (commercial kits avaliable, eg Thermo Mouse Regulatory T Cell Staining Kit, CatNo 88-8118-40 )?Alternatively, do we have to differentiate Treg step by step like below: Peritoneal lavage fluid - leukocytes - mCD45+ - mTCR beta + - CD4+ CDfoxp3+ ?(also shown in attached picture).
Thanks so much. I am new in Flow. It makes me confusing.....

I'm gonna to make arthritis model induce by serum from K/BxN. However, K/BxN mice are unable to breed. So where can I purchase K/BxN serum?
Hi everyone,
I have been struggling with the differentiation of mouse SVC cells into mature adipocytes lately.
In the past, I have always isolated and cultured SVC in 15% FBS HG-DMEM and induced differentiation using an MDI cocktail (no rosiglitazone added) (0.5mM IBMX, 2uM Dexamethasone, 10ug/ml Insulin), the result was always good with ~ 80-90% cells showed adipocyte phenotype, and lipid droplet formation.
However, recently, all of my cells are not able to differentiate anymore, regardless of all of my attempts:
1. Younger mice: I usually use 3 weeks - 5 weeks old male mice
2. Refresh of chemical stock: I have refreshed and bought all of the MDI components
3. Seeding: I've tried seeding on all 6 wells, 12 wells, and 24 wells plates with low and high confluency
4. Adjust FBS level: although my previous results were just fine with 15% FBS, I have tried 5% and 10% after having the problem but it was not solved
I have always had a good amount of viable cells after isolation following this protocol:
I am searching for help and suggestions, please leave a comment if you have any experience with the same issues or if you have some solutions for it.
Thank you all!!!
Hi,
I have having an issue with the Fc blocking stages when carrying out flow cytometry on a murine macrophage cell line. I am using a rat anti-mouse antibody against the scavenger receptor MARCO. Unfortunately, I have been unable to find a conjugated version of this antibody and so I have a secondary goat anti-rat F(ab')2 to use with it. I have an Fc blocking solution, however this is also raised in rats and causes non-specific binding as the F(ab')2 fragment binds to this blocking solution as well as the primary antibody. I have been unable to find any Fc blocking solutions that are not raised in rat and I have tried various serums to try and block the Fc receptors instead, but have been unsuccessful (I have used a variety of mouse, goat, and FBS combinations).
I was wondering if anyone knows of any Fc blocks that are not raised in rat I could try, or whether there is any advice on how to get good blocking results using serum?
Thanks very much!
in the NIH test for checking the potency of the rabies vaccine, we must make dilutions. In the second dilution, we have a sharp drop and the mortality of mice. I wondering if my dilution technique is incorrect. I use a sampler for dilution. is it a different method for dilution of vaccine with adjuvant?
I am trying to conduct the Morris Water Maze , but it seems that the mice do not want to reach the platform. what would be a possibe cause ?
when they reach the platform they sometimes stand on it for a few seconds then jump off and continue swimming , and other times they just walk across the platform and fall off.
anyone faced a similar issue or has any ideas please ?
Hi everyone,
I tried many published protocols to induce type 2 diabetes in c57 female mice using high fat diet for 1 month then STZ 40 mg for 4 days or 50 mg for 5 days followed by 10% sucrose in drinking water to avoid hypoglycemia however, the mice were resistance when I gave 40 mg stz for 4 days and died when I inject 50 mg for 5 days (these 2 protocols were used in multiple papers including nature ones). Any suggestions?
Thank you in advance
Sherif
i read many papers that used the invitro drug release test but never showed the details of how they calculated the dose from it.
I am working with C57BL/6 mice for the development of a T2DM mouse model. During the course of this experiment, the mice will have their urine, blood, and tissue samples collected and examined for the presence of specific biomarkers. These biomarkers will need to be accurately quantified. At present, I understand ELISA to be the gold standard for this, but are there any alternative assays that can be done?
I am treating mice with doxycycline (75ppm) to induce gene expression but i see dermatitis in both control and transgenic mice.
Hi there!
For a while now I have been looking for a review or textbook or research papers that very specifically outline the nuances of human and mouse brain development in the cortex, specifically in the ventricular zone. There are differing nomenclatures as well as subtle cell types or cell type behaviours, but I can't seem to find any specific resources comparing the two. It would really assist me in deciphering some nuances in my thesis experiments.
If anyone knows of a paper comparing the two, or even 2 articles each about human or mouse that are specific I would very much appreciate them!
"How do we understand special relativity?"
The Quantum FFF Model differences: What are the main differences of Q-FFFTheory with the standard model? 1, A Fermion repelling- and producing electric dark matter black hole. 2, An electric dark matter black hole splitting Big Bang with a 12x distant symmetric instant entangled raspberry multiverse result, each with copy Lyman Alpha forests. 3, Fermions are real propeller shaped rigid convertible strings with dual spin and also instant multiverse entanglement ( Charge Parity symmetric) . 4, The vacuum is a dense tetrahedral shaped lattice with dual oscillating massless Higgs particles ( dark energy). 5, All particles have consciousness by their instant entanglement relation between 12 copy universes, however, humans have about 500 m.sec retardation to veto an act. ( Benjamin Libet) It was Abdus Salam who proposed that quarks and leptons should have a sub-quantum level structure, and that they are compound hardrock particles with a specific non-zero sized form. Jean Paul Vigier postulated that quarks and leptons are "pushed around" by an energetic sea of vacuum particles. 6 David Bohm suggested in contrast with The "Copenhagen interpretation", that reality is not created by the eye of the human observer, and second: elementary particles should be "guided by a pilot wave". John Bell argued that the motion of mass related to the surrounding vacuum reference frame, should originate real "Lorentz-transformations", and also real relativistic measurable contraction. Richard Feynman postulated the idea of an all pervading energetic quantum vacuum. He rejected it, because it should originate resistance for every mass in motion, relative to the reference frame of the quantum vacuum. However, I postulate the strange and counter intuitive possibility, that this resistance for mass in motion, can be compensated, if we combine the ideas of Vigier, Bell, Bohm and Salam, and a new dual universal Bohmian "pilot wave", which is interpreted as the EPR correlation (or Big Bang entanglement) between individual elementary anti-mirror particles, living in dual universes.
Fred-Rick Schermer added a reply:
Abbas Kashani
A lot to work with, Abbas.
However, I am standing in a completely different position, and want to share my work with you. I hope you are interested about this completely distinct perspective.
My claim is that Einstein established a jump that is not allowed, yet everyone followed along.
Einstein and Newton's starting point is the behavior of matter through space. As such, one should find as answer something about the behavior of matter moving through space, and yet Einstein did not do that.
To make the point understandable quickly, Einstein had not yet heard about the Big Bang yet. So, while he devised his special relativity, he actually had not incorporated the most important behavior of matter through space.
Instead, he ended up hanging all behaviors of matter on spacetime. It does not matter that his calculations are correct.
--
Let me find a simple example to show what is going on.
We are doing research on mice in a cage, and after two years we formulated a correct framework that fully captures all possible behaviors of these mice in the cage. That's the setup.
Now comes the mistake:
The conclusion is that the cage controls the mice in their behaviors.
Correctly, we would have said that the mice are in control of themselves, yet the cage restricts them in their behavior. We would not say that the cage controls the mice.
Totally incorrect of course, and yet that is what Einstein did. He established a reality in which matter no longer explains the behavior of matter through space, but made it space (spacetime) that explains the behavior of matter. It is a black&white position that has to be replaced by the correct framework (which is a surprise because it is not based on one aspect, but on both aspects).
--
I know I am writing you from a perspective not often mentioned, and it may not interest you. I'll find out if you are interested in delving deeper into this or not.
Here is an article in which I delve into this matter more deeply:
Article On a Fully Mechanical Explanation of All Behaviors of Matter...
This is the first time I did an H&E staining, and I found that there are some white (blank) spaces between the cells. I do not think this is the normal morphology of this tissue.
Here are two screenshots of the tissue. They are from exactly the same microscope slide, both of them are mouse heart tissue, and I think most of the cells in the picture are cardiomyocytes. They are 2 consecutive slices from a frozen cryotome.
My own opinion is:
1. I don't think these are adipose tissue.
2. In both screenshots the longitudinal muscle (top part) looks alright. The transverse muscle (bottom part) in picture 1 has white spaces, while the transverse muscle (bottom part) in picture 2 is normal. I am wondering if it is because some of my operations has damaged the tissue? and the transverse muscle is more prone to the damage(?)
This is the protocol that I followed:
1. Do fresh frozen sectioning;
2. Submerge the slide in 100% MeOH at -20 degree celcius for 30 min;
3. Remove slide, let MeOH evaporate;
4. Stain with hematoxylin for 3 min, wash by dipping in beaker with water for 15 times. Repeat with another beaker of water.
5. Stain with buffered eosin for 1 min, wash by dipping in beaker with water for 15 times. Repeat with another beaker of water.
6. Airdry.
Could anyone please tell me what might be wrong?


I am wondering if this is a technical issue that occured during processing. I have murine femurs that were decalcified in 15% EDTA over 2 weeks and then processed and I embedded them into FFPE. Before I started to section the bones I noticed what looked like air bubbles around the bone. I melted the blocks and determined that the air pockets are actually within the shaft of the bone (2-3mm in length). Does anyone have any suggestions on how they might have gotten in there and if there is a way of removing them without damaging anything?
Dear scientists, we are currently conducting a mouse model for wound healing. The process is as follows: make the wound, apply and suture the silicone splint, apply the tegaderm and apply the treatment topically every two days. Our problem is that after 6 days, the mice start removing the silicone. Is it common for silicone to be removed? Would it be possible to put it back on or put another one on? What could we do to avoid this? Thank you so much!
Hello, I am looking for a protocol to sterilize olive oil that will then be suitable to gavage to germ free mice. Importantly, if it were to be dry-heated, could it pose a fire risk?
For a project I'm included in I'm desperately in need of audio recordings of male mice. They need to be original files recorded with the correct equipment.
I need them for research on gait ataxia in mice, sounds of male mice could motivate female mice to move from point A to B which is needed for me to analyse possible gait ataxia.
Thank you in advance!
I am planning an immunoprecipitation experiment using Mouse monoclonal [11C9] to Mannan Binding Lectin/MBL (ab26277) antibody to immunoprecipitate for MBL2 in human serum. There are many protocols online for immunoprecipitation utilising cell cultures, where the recommended amount of cells tends to be 10^6 to 10^7, however not so many that utilise human serum.
Reading online, the optimal total protein load of immunoprecipitation seems to be 1-5 mg/mL, with 0.1 mg/mL being the minimum recommended load. Considering that the normal range for total protein in human serum is 60-83g/L (average: 71.5 g/L), loading 5 mg of total protein for immunoprecipitation would mean I need 69.9 mL. Alternatively, loading the minimum (0.1 mg), I would need 1.395 mL of human serum based on my calculations.
I cannot afford to be using 1.395 mL of human serum in my experiment due to lack of sample volume. I was wondering if anyone can share the amount of human serum they've used for immunoprecipitation before, where it was successful. Thank you in advance.
In some gene editing studies using mice, the delivered plasmid carrying the editing tool (e.g. TALEN) also carries both an HA-tag and GFP. The GFP is separated from the editing tool with a T2A sequence. Why are HA and GFP both needed?
Hello everyone, I am seeking guidance on the analysis of scanned films obtained from Proteome Profiler Mouse Cytokine Array Kits, specifically focusing on the quantification of dot intensity using ImageJ. I have successfully scanned the array membranes, which contain dots exhibiting varying intensity levels, ranging from intense to very light. However, I am facing challenges in determining the optimal method for selecting the appropriate area for intensity measurement, particularly due to the variability in dot intensity across the membranes.
I am uncertain about the most suitable approach for measuring dot intensity and comparing them across the membranes. Considering the diverse range of dot intensities present on the membranes, I am unsure whether to fix the area for intensity measurement or adopt a different approach.
I would greatly appreciate guidance or recommendations on how to effectively analyse the scanned films to quantify dot intensity using any available protocol or instruction using ImageJ. Specifically, I am seeking insights on selecting the appropriate area for intensity measurement, taking into account the presence of both intense and light dots.
I have attached a sample image of the scanned film for reference, and I am more than willing to provide additional details or clarification upon request.


We need help from people who have contracted mouse xenograft model with HCC1954 cell line. When 1x10e6 cells/mouse (50% matrigel) cells are scanned in a 6w female mouse, a tumor volume is formed about 100mm^3 after about 3 weeks. After that, the growth rate of cancer is jagged. The tumor tissue is not solid(hard), and the inside of the tumor continues to show symptoms of water filling. I tried about 5 times for animal efficacy experiments, but these problems still appear. I'm looking for advice if you've done efficacy experiments with this cell line.
To what amount can you dilute matrigel? It to inject PDX subcutaneously into mouse. Undiluted it's almost impossible to work with, but overdilution leads to matrigel being incapable to solidify. What are the best practices?
Hi everyone! I am used to doing BCA test while doing protein extraction from cells or tissue, but do you usually use it while working with serum, i.e. mouse serum? Or do you just consider your results from ELISA or dot blot according to 1 ul of serum?
I've been able to activate mouse T cells to make CARs fine the last few months, but the T cells seem to not be activating as nicely and therefore are dying (even the non-transfected controls) and not expanding.
After two days of CD3/CD28+IL2 beads they barely expand and then after removal of beads and culturing for 2 days (with IL2) , they all die.
I have made new T cell media everytime - so I don't think that is the problem. I did start using new IL2 and new beads, but I have also tried multiple boxes and vials and still its poor.
Let me know if you have any suggestions on what to do or tips to improve as culturing mouse T cells has been a struggle for me the last year!
I want to do mathematical modelling of Pharmacokinetic data from plasma drug concentration in mice. Can someone please suggest me any freely available software for PK Modelling.
I'm planning an experiment where I can access intracellular cytokines in a specific subregion of the brain in mice. However, this brain region is quite small, maybe 50,000 cells per animal. I know I will need to pool mice but how many would I need to pool? Can I use 500,000 cells? Pooling more than 10 mice wouldn't be feasible.
Thank you!
I want to do stereotactic surgery/ microinjection in post-natal/juvenile mice within the age range of P10-P16. I am specifically looking for suggestions on anesthesia. The pup does not get anesthetized with 1% Isoflurane (0.8-1 litre/min oxygen flow). Induction was observed at 2% but trying to maintain the anesthesia at 1% leads to death. Below this level, pup does not get anesthetized. I don't want to work with Hypothermia induced anesthesia. So, if anyone has any experience in these type of surgeries, please suggest any tips or tricks to anesthetize the pups in this age range.
Hello Dear Community
Does somebody has a protocol to dissolve the GSK126 at a concentration of 12 to 15 mg/mL in DMSO and Captisol?
Thank you by advance for your help
Christophe
Does anyone know any protocol I can follow for muscle fiber-type staining for paraffin-embedded tissue?
Dear scientists, we are currently carrying out a wound healing mouse model. The process is as follows: make the wound, apply and suture the silicone splint, put the tegaderm and apply topically the treatment every two days. Our problem is that it's very difficult for us to remove the tegadem and we are hurting a little bit the mice when we do it. In addition, sometimes mice also remove the tegadem. We have tried putting an elastic bandage on it but it doesn't work either. Does anyone have these problems and can tell us the best way to cover and remove the tegadem without having it removed or causing damage? Thank you so much!
I want to study depressive like behavior associated with LPS, which is rather expensive.
Hi guys,
I'm working on the tumor mouse model. In general, I prepared tumor cells and injected cells into the flank of mice subcutaneously.
After around 3 weeks, I found, in the same group, there's a variation in the tumor weight. For instance, some tumor were around 1.2g, some 0.6g.
Could you give me some suggections or tips about how do tumor inejction?
Which type of needle do you need for injection? or How to prepare tumor cells suspension? or whatever something you think is important to do that successfully
Many thanks :)
lease help me to learn ImageJ software to analyze the immunoflourescence images. i need to analyze cortical neurons in the brain of stroke model of mice.
I am seeking advice in identifying reliable and specific markers for accurately detecting tumour regions in colorectal cancer mouse cryosections?
Any recommendations or insights would be greatly appreciated. Thank you
Hi, I' am trying to make stable cell line using plasmid transfection.
the plasmid are available for neomycin selection.
I want to know the appropriate G418 concentration and time forselection about Mlg cell line. Mlg cell line is mouse lung fibroblast cell line.
and also I wonder the concentration and time of selection about mouse primary lung fibroblast.
Thank you
I am using mouse serum to block Fc receptors before staining, for flow cytometry. My question is if I should also add the serum to compensation beads before staining them, so that the cells and the beads go through the same processing.
Thank you very much in advance!
Dear all,
when I analyzed a list of genes mutated in our mouse tumor model in Enrichr, the Users Contributed List 'Sca1+genes' overlapped perfectly, but I cannot find any information about the origin of this gene list.
Can anyone help me? Thank you.
Hi! I am trying to do RNA extraction from p7 mice's somatosensory cortex and hippocampus. But I am not sure if the RNA was extracted from the correct brain region. I was wondering if there are any standard ways to test it. Thank you.
Have anyone tried to measure calprotectin from feces sample of mice using ELISA
which is best rodent for oral sub acute toxicity ? rat/mice
I am treating tet on mice with doxycline (76ppm) for 2 weeks and i dont get the overexpression according to Western Blot. In rtPCR i see differences, but in protein level these differences are not reflected. What might be the issue?
Hi all. I'm wondering what everyone's thoughts are on automated cell counters for use with mouse lymphocytes. I have used the Countess II FL in the past, but found it to be less than ideal as it was difficult to optimize for use with lymphocytes. I found the NC-200 and I'm curious if anyone on here has tried it for lymphocytes?
I have experimented to assess the T-cell memory status in mice models. However, I am unable to separate the CD3+ T-cell cluster from the splenic cells using different anti-CD antibody markers, specifically CD3 (Picture attached here). I followed the standard protocol for sample preparation and staining except used a 1 mL pipette-tip instead of a syringe with a 22G needle to make a single cell suspension. Now my question is how can I solve this problem? Thank you in advance for your consideration and valuable suggestions.

This vibratome seems to have a great reputation, however, I am struggling to find if anyone has used it to record from adult Purkinje neurons in acute brain slices (from mouse). Compared to many other cell types, Purkinje neurons are tricky to keep alive and record from. The studies I have looked at (with Purkinje neuron recordings) utilize leica vibratomes. I am considering the leica 1200S and the VF-310-0Z. It's amazing that the VF is less than half the price of the Leica 1200S. If you have any experience in this regard, please let me know!
Thanks!
Joey
I am doing some mice work and one protocol is doing subcutaneous injection on the tail. I didn't find any video for this online. Anyone has ever did this?
I am performing western blotting using tissue lysates prepared from white adipose tissues isolated from mice. The targets are membrane proteins. The protein quantification was carried out using BCA. I have attached the protein conc results.


I got this plasmid from Addgene (https://www.addgene.org/83481/) that I want to put into some mouse immortalized myeloid cells.
The basticidin resistance gene is driven by human phosphoglycerate kinase 1 promoter (hPGK). Will this be a problem?
The images below show mice fetuses on prenatal day 15. However, I noticed some swellings that looks more translucent in some cases. I want to know if it is a classical abnormality or just sign of growth delay? Thank you for your contributions.





I need to study the relationship between gene expression of GDF9 GENE in mice and its relationship to their exposure to heavy metals, AND please what about recommended me about this study
I study diabetic tendinopathy and would like to know the reasons for choosing rat animal models over mice when it comes to metabolic syndrome. Your help and discussions are very valuable to me. Thank you.
I am looking into selecting T cells with microbeads for isolation and subsequent adoptive transfer in immunodeficient NRG mice. I am hoping to do positive selection with microbeads. After column selection, I am wondering how the T cells will act in vivo after transfer. Do antibody/microbead-bound T cells act differently or are eliminated in immunodeficient NRG mice?
I work on mouse Tibialis Anterior (TA) muscle tissue and encountering crystal formation issues with OCT usage during frozen sectioning. Could anyone share a concise protocol and tips to prevent crystal formation in this process?
Thank you!
In my experiment, I activate a creER mouseline using tamoxifen. The mouse is firstly injected with a cre-dependent virus in the brain, then after several days, tamoxifen is injected i.p. to activate creER.
My questions are:
1. How many days after the virus injection should I inject tamoxifen to activate creER?
2. If I inject tamoxifen for 5 consecutive days, how long can the virus-labeled cells be activated for? My experiment needs long-term monitoring for at least 4 weeks.
Thanks!
I am doing ICC to check for colocalization of my protein of interest and mitochondria. Without having to use Mitotracker, (I'm currently have issues with finding the optimal concentration) or buying a mouse antibody for one of the proteins, how can I work around co-labeling using primary antibodies of the same animal (right now we have rabbit for both proteins).
Hello! I was working with mice primary cell cultures to evaluate in vitro cytotoxicity of a treatment, I have an n of 10. The problem is that I don't know what technique to use because when I try to do it with Mann-Witney the program gives me an error
Are there methods to measure mice length without giving anesthesia? Mice keep moving when you try to put them against a table with measuring tape, so the normal pratice is to give anesthesia. However, Anesthesia is costly, risky and may have confound the results of blood tests in mice.
Hi, I am new to this platform. I am currently preparing for Western Blot experiments using Young (7-10 mo) and Aging (20+ mo) mice and the protein of interest will be tight junction proteins (ex. occluden, claudin-5, ZO-1).
I have looked through a series of commonly used housekeeping genes (e.g. β-actin, α-tubulin, β-tubulin and GAPDH) but found that all of them will be affected by aging. I have also looked through stain-free and total protein normalization (by Ponceau S or Coomassie staining), which seems to give a more promising result than the housekeeping genes. But since they are relatively new approaches, I would like to seek opinions here about a good way for the loading control of age-related Western Blot.
Thank you very much!
I am to collect mice fecal samples for microbiome analysis. I've been reading some articles where each mouse was placed in a cage and allowed to defecate for a few hours since it is ideal to use fresh stools for high yields of DNA.
However, I am worried that some mouse might not defecate or the stools may not be enough. I am thinking of placing each mouse in an individual cage overnight and collect the fecal samples in the morning. Can you recommend this method? What method can you recommend to allow collection of sufficient mouse stool sample while ensuring preservation of stool microbial DNA?
Hi guys,
We introduce some mice with two adjacent point mutation of specific molecules, however, it is difficult to carry out genotyping of this mice.
DNA base pair and protein size is similar to original molecules. In this situations, how can i perform genotyping to this mice?
Do we have to DNA sequencing every mice?
I look forward to hearing the opinions of experienced individuals., thanks.
I have a mouse skin wound healing model, and it's suggested to give pain management after wounding procedure. It's known that carprofen is a nonsteroid anti-inflammatory agent, acts as a multi-target FAAH/COX inhibitor. Can I use carprofen as a pain reliever after mice surgery when inflammation is an potential effector in my study? If not, could you advise on some appropriate medications?
I am using a Tet on system to overexpress a LncRNA in mouse ES cells, based on a piggybac vector (pXlone). I performed transient and stable experiments. In both cases, the LncRNA is expressed even without adding dox in the culture medium. Can someone help me on this ?
Thank you very much.
I am performing stereotaxic injection of an AAV8 serotype into mice and then immunofluorescent staining for target antigens in free-floating brain tissue. The surgeries appear to go very well and the mice recover normally. However, when I complete the immunofluorescent staining of my target antigens, there is an *extremely* high fluorescent background signal and a complete (or nearly complete) absence of detectable specific signal for my antigen, but only in the vicinity of my injection site (e.g. if I inject into the cortex, the problem is localized to the cortex but not deeper areas; if I inject into the NAc, the problem is specific to the NAc but not the cortex)
Procedure: I perfuse ~4 weeks post-surgery via transcardiac perfusion with saline (0.9% NaCl in ddH2O) and 4% PFA, followed by an overnight post-fixation with 4% PFA. The brains are cryoprotected in 15% then 25% sucrose and frozen on dry ice, then sectioned at -20C on the cryostat. Tissue is permeabilized in PBS-T (0.3% Triton X-100) and blocked with normal serum for the same antibody (5% up to 10%). I have tried incubation in primary antibody overnight at +4C and longer periods (3 days at +4C up to 7 days at +4C). Secondary antibody has been tested at several concentrations and for either 1, 2, or 3 hour incubation periods (RT, shaking).
My initial suspicion was some kind of contamination/infection resulting from the surgery/capillary, however, I have been extremely mindful of keeping everything as sterile as possible and the problem persists across numerous surgeries and litters. I am now wondering if the problem could result from an immune reaction to the virus itself, or another issue. This problem doesn't seem to occur in all mice, but does in a significant portion of them.
If anyone has encountered similar problems or has suggestions for troubleshooting, any help would be greatly appreciated :)
Someone had told me 3-5% in drinking water but I can't seem to find a reference how much DMSO is OK or too much.
I made a kind of gene knock-in mice, the vector was inserted after the endogenous promoter of this gene. For the vector, it includes part of exons of WT gene and the WT fragment is flanked by two loxP sites following which there is a part of exons containing amino acid mutant(one amino acid is mutated to another). So this gene-KI F/F mouse expresses WT gene and it expresses gene mutant in which case it breeds with Cre mice. Is there any method to detect the gene expression of this mutant? Western blot cannot work because the length of this gene is consistent......
I have to treat the mice with a drug that is dissolved in DMSO, but to decrease the percentage of DMSO I want to add PEG400 and cyclodextrin.
I would like to know the maximum recommended percentage that I can use of PEG400 when injecting IP in mice.
I am using SOX1 and Nestin as primary antibodies (1:200 and 1:500 for each) and Anti Goat Alex Fluor-594 and Anti Mouse Alexa555 as secondary antibodies (1:1000) and still not getting any results. Please help me with the concentration of primary and secondary antibodies or any other suggestions if I am doing something wrong. Thank you!
I want to conduct haemolyis assay on mouse blood (I need 0,5 - 1 ml of blood), I know that it is generally hard to collect good sample without inducing haemolysis. As a anticoagulant do you prefer EDTA or heparin? Syrgine must be coated with anitcoagulant? And need I special tubes that are coated with anticoagulant or can I just prepare solution of anticoagulant in a tube? As I understand blood should be collected from heart or junglar vein? Do you have any recommendations?
I am inducing Ethylene Glycol to the mice to grow urolithiasis and confirmed it by the urine samples by 28 days interval. Now my aim is to know if any increase in volumw is happening by inducing them desired plant extracts. Then i want to measure the actual stone size formed by EG.
I am performing plaque assay from the spleen of mice infected with virus. I am facing a problem that in few mice i am getting plaques but in other mice samples i am not getting any plaque or less plaques in comparison to other mice of same group. I am not able identify the problem. I am washing spleen with pbs after collection and immediately storing this in liquid nitrogen. After this I am homogenizing spleen and performing plaque assay. Any help or suggestions will be appreciated. Thanking you in advance.
Does anybody know how many endothelial cells are present in the heart of an adult mouse?
What should be a valid explanation for including only male mice in depression studies (Earlier estrous cycle phase hormonal changes was one of the reason for excludinng female mice, which is not acceptable in present time)
We have a protocol that has our mice running 3 hr/day. However, our Exer treadmill has some features that do not promote the encouragement of their running. In pic #1, you can clearly see a significant gap between the treadmill and the (non functional) shock grid - we tried putting test tubes in to fill the gap. I cut a thick (maybe too thick?) wooden dowel to fit in the gap, but that poses a problem because it is a perfect place for them to perch when they're tired. I then tried placing a dowel before the gap, but they still find a way to perch on it.
Does anyone have an idea on how to encourage running on this treadmill, and/or know a way to prevent them from falling in the gap? Thanks in advance to anyone reading, I encourage any creative ideas

Dear all,
in humans I believe it is widely accepted to gate Tregs as only CD4+ CD25+ and CD127low/- despite intracellular staining of FOXP3 certainly being the most precise method to do so. I´ve been trying to find any evidence if this surface marker combination can also be used to gate mouse Tregs or if FOXP3 staining is necessary.
It would be very much appreciated if anyone could report about their experience or even provide publications that have proven this procedure to be scientifically adequate.
Cheers!
Richard
Can we use the kit for mice to study inflammation activities in fish for TNF alpha, IL-beta and iNOS in serum
In other to evaluate effects of a natural compound brain ischemic stroke . So we need brain ischemic stroke mice model.
Is there any one to help about this?
I am a doctoral student in Iran and I need the following materials for my doctoral thesis:
VEGFR2 primary antibody
VEGFR1 primary antibody
Mouse secondary antibody conjugated to fluorescein isothiocyanate and phycoerythrin
Mouse secondary antibody conjugated to HR
VEGFR3 primary antibody
bFGF primary antibody
VEGFC
Unfortunately, due to the embargo and the high price of these items, it is not possible for me to complete my thesis.
I kindly request your guidance in this field and any suggestions you may have. Is it possible for me to collaborate with professors outside of Iran? Can I attend foreign universities with full funding? Furthermore, do you know any supporting institutions that can assist me in this matter? If so, I would greatly appreciate any introductions you can provide.
Thank you very much.
What should be a valid reason to use male mice for depression studies and excluding female mice.
Hello!
We are generating conditional knockout mice using CX3CR1 Cre for a particular gene. During this process, we are performing tail cuts for genomic extraction and genotyping. We've designed primers for flox PCR to detect a deletion band if Cre is present. However, we've encountered an issue. Despite the tail typically having fewer cells like those containing CX3CR1 Cre (such as microglia, macrophages, dendritic cells), deletion bands are occasionally detected in tail genotyping. In some mice, these bands appear stronger than the normal flox allele bands. Conversely, in other mice with Cre, no deletion bands are observed at all. Could this be due to low-level expression of CX3CR1 in some cells in the tail? Should germline depletion be considered in these scenarios?
Thanks,
The mice were induced to have type 2 diabetes by feeding high-fat food and injecting low doses of STZ.
I am new to the field and I am currently working with lenvatinib. I have found that different research groups used different vehicles for lenvatinib in mouse experiments. I have even found out that some research groups did not mention the vehicle they used. I am so confused. Which vehicle should I use? For example, one group of researchers used water to prepare 3 mg/mL lenvatinib solution. (Mizuo, H., Mano, Y. Cross-species comparison in nonclinical pharmacokinetics of lenvatinib by a simple HPLC with ultraviolet detection. Sci Rep 13, 8349 (2023). https://doi.org/10.1038/s41598-023-35297-z). I tried it, but the powder did not dissolve. Can anyone help?
Is there any specific reason not to change strain of the mice for the same cell line?
I have collected mouse fecal matter and stored at minus 80 degree C. I want to culture the gut microbiome in fecal matter and then will do MALDI-TOF MS for speciese and strain identification.
Can I do the culture from Frozen Fecal matter Samples?
1. tumor cell stock : 2.8 x 10^6 cells per 1 mL (Cell concentration = 2.8 x 10^6 cells/mL)
2. mice number : 2 mice
3. tumor cell number and volume when doing injection to a mouse: 1 x 10^6 cells per 0.015 mL
I have no idea which calculation is exactly right, but I guess the first explanation is correct.
The first calculation applied a proportional formula, and the seconde calculation applied a formula, which is 'concentration x volume = concentraion' x volume''.
The first calculation is
2.8 x 10^6 cells : 1 mL = 1 x 10^6 cells x 2 mice : x mL
x = (1 x 10^6 cells x 2 mice) / (2.8 x 10^6 cells) = 0.714 mL (714 uL)
Thus, I cannot make 1 x 10^6 cells in 0.015 mL. I should subculture the tumor cell again.
The second calculation is
2.8 x 10^6 cells x x mL = 1 x 10^6 cells x 2 mice x (0.015 mL x 2)
x = (1 x 10^6 cells x 2 mice x (0.015 mL x 2)) / 2.8 x 10^6 cells = 0.021 mL (21 uL)
Thus, I prepare 1 x 10^6 cells in 0.015 mL for 2 mice by mixing 21 uL cells and 9 uL PBS.
Which one is correct?
Would you please check the calculation?
Hi everyone,
I aim to isolate MSCs from the bone marrow of the mouse. I put the bone marrow cells into the cell culture flask containing DMEM low glucose+BFGF+vit C.
I know that now I have lots of different cells inside the flask but this type of the cells that I posted the picture are growing in a line and I am so curious to know what is the type of these cells. Does anyone have an idea?

I have two groups of mice representing WT and knockouts. Each mice group (WT and KO) will be allocated to 4 experimental categories. I need to allocate these available mice into these experimental categories, with no intra-category and inter-category significant differences in their age and weight. Is there any accepted way to group these mice by matching their age and weight?
I’m trying to make a solution of metronidazole to add to drinking water of mice. I’m making a 100mg/mL solution but it won’t dilute. I need to filter sterilize the solution through a 0.2uM filter. I would love it if I can make it to 300mg/mL.
any suggestions?
Any procedure or video would be helpful.
Hi,
I am running some mouse serum samples in western blot, using regular loading buffer. I would like to find a housekeeping gene (in the serum) which I can normalize my results to, in order to verify that the loading is equal. Other methods besides housekeeping genes are welcome as well. thank you
I need to get the mouse MSC from bone marrow to analyze the expression of a specific gene in KO and wild type. Including the 3 lineage differentiation.
We want to study the effect of High Fat Diet in rats.......
Hi,
I'm working on the immunity of tumor. I injected tumor cells into the mice subcutaneously. After harvesting the tumor, I used percoll and isolated immune cells. Then I added trypan blue and counted cells using a cell counting chamber before flow cytometry staining.
I found in most of the papers, they just said "Count cells for staining". But they didn't say count which kind of cells. Because I saw there were different type of cells in the microscope.
Do I need to count all living cells, no matter how different the shape they are. Or do I just count one specific cell?
Many thanks.